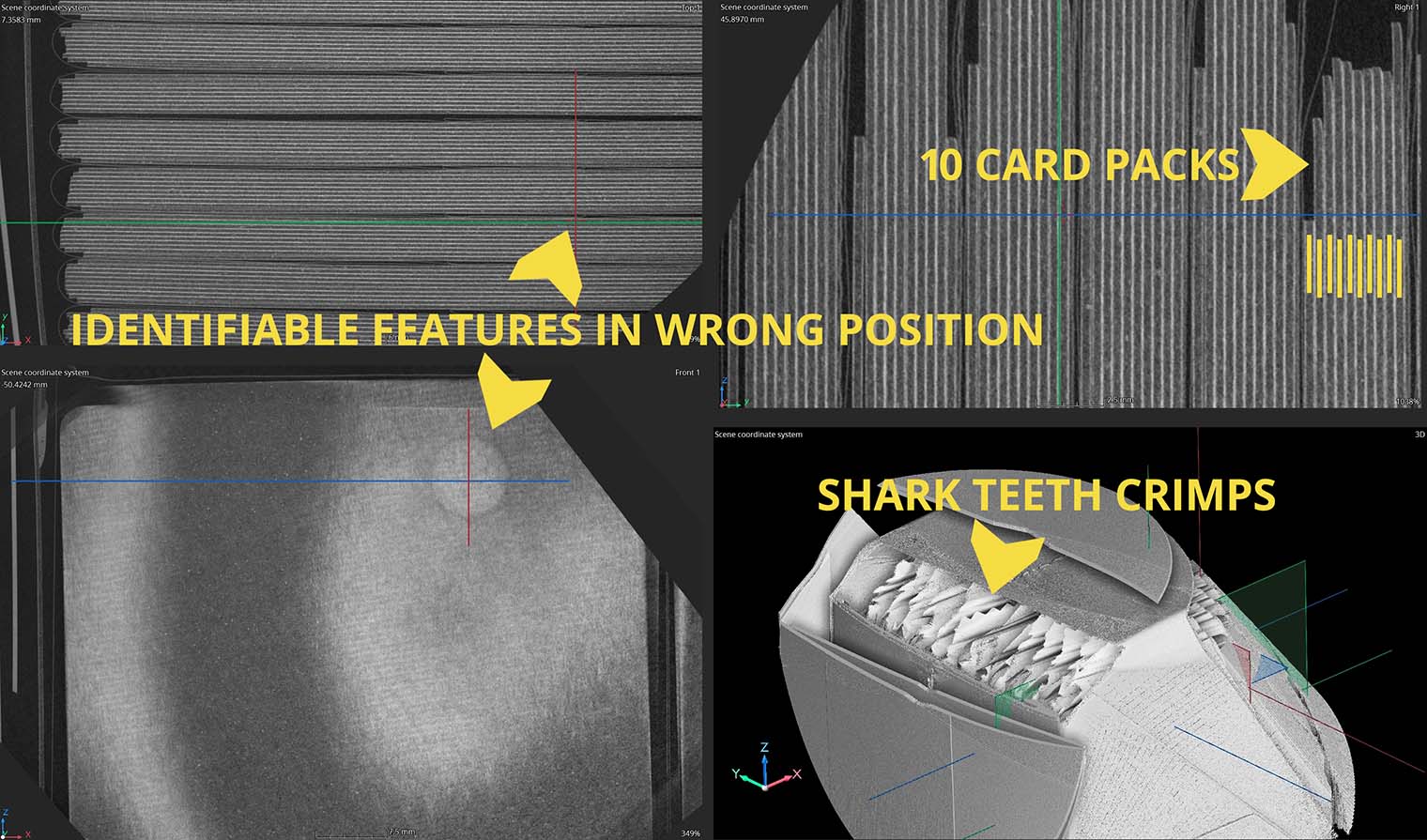
DYE PENETRANT TESTING OF MISSION CRITICAL COMPONENTS
Industrial’s team of exceptionally qualified Nondestructive Testing Experts, like Steven Prozinski & Megan Davey, ensure the safety, reliability, and efficiency of critical assets.
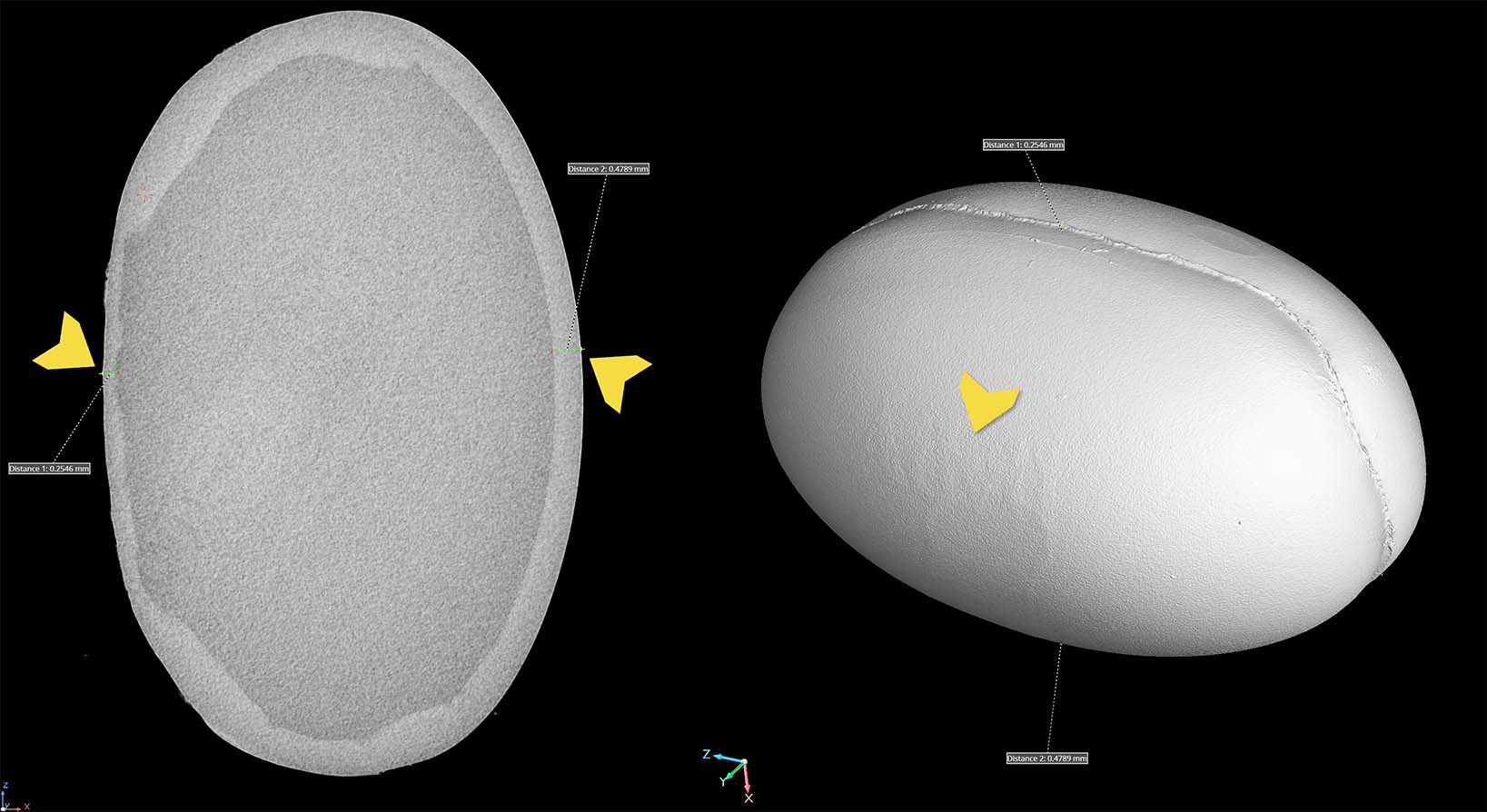
This case study offers a glimpse into the practical application of liquid dye penetrant using fluorescent sensitivity level 3 penetrant and dry powder developer. The test piece is a helicopter lower bearing support casting. Penetrant testing involves applying a liquid dye (typically a colored or fluorescent penetrant) to the surface of a part. After a specified dwell time, excess dye is wiped off, and a developer is applied. The developer helps to pull out the dye from any cracks or surface flaws, making them visible to the inspector.

This standard and black lighting comparison demonstrates the importance of performing this type of nondestructive testing. Under standard lighting there is little to no visual evidence of cracks at the supports. Fluorescent dye under black light shows that the cracks are severe.


Wipe tests are used to verify indications. In a wipe test, the technician uses a q-tip or wipe to remove the excess dye, and then re-examines the part for bleedback. The below video demonstrates extreme severity of the crack-like indications because the indication didn’t appear to be wiped away.